At the beginning of this year, I was among those celebrating a groundbreaking decision by CMS: the agency said it would begin covering the use of certain continuous glucose monitors (CGM) for people with insulin-requiring diabetes.
But a more recent ruling by CMS is nothing to celebrate. According to this ruling, “if a beneficiary uses a non-DME (durable medical equipment) device (smartphone, tablet, etc.) as the display device, either separately or in combination with a receiver classified as DME, the supply allowance is non-covered by Medicare. In other words, Medicare will not reimburse for CGM-maker Dexcom’s G5 smartphone app, and people who wear the company’s glucose monitor are being told they can’t use Dexcom's "share" function that enables one person—a family member, say—remote access to another’s glucose levels to make sure they are safe throughout the day.
CGM’s sharing capability is the type of innovation that can head off adverse events and save lives, especially among elderly people with diabetes who are at increased risk for recurrent and/or symptom-free hypoglycemia. CMS’s decision seems heartless to me at worst, short-sighted at best. It reflects a lack of understanding about the future of digital health and stifles innovation in an industry where smartphone integration is truly essential to making life easier for people with intensively managed chronic disease.
As a result, I will be joining medical technology innovators and advocacy groups to lobby for the reversal of this CMS decision. It’s a setback for remote monitoring of chronic health conditions in general, and also seems to suggest that the smartphone has no place in the world of medical technology. I could not disagree more strongly with this premise.
CMS remains far behind the curve as mobile devices and remote monitors develop new capabilities, functions that will make disease management more convenient for patients and their families.
Some have been saying CMS is worried that allowing glucose readings to be accessed remotely via smartphone will mean the agency will be required to buy smartphones for Medicare beneficiaries. This is not going to happen because this was never the point in designing a solution to be used with a smartphone.
In the case of Dexcom’s glucose monitoring systems and insulin delivery systems like the ones we are designing at Bigfoot Biomedical, the smartphone presents an easier way to view information, utilizing a supercomputer most of us are already carrying in our pockets. Disallowing its use means people with diabetes will have to carry around yet another gadget or perhaps neglect to monitor their blood glucose because of cumbersome, complex, and antiquated processes required to do so.
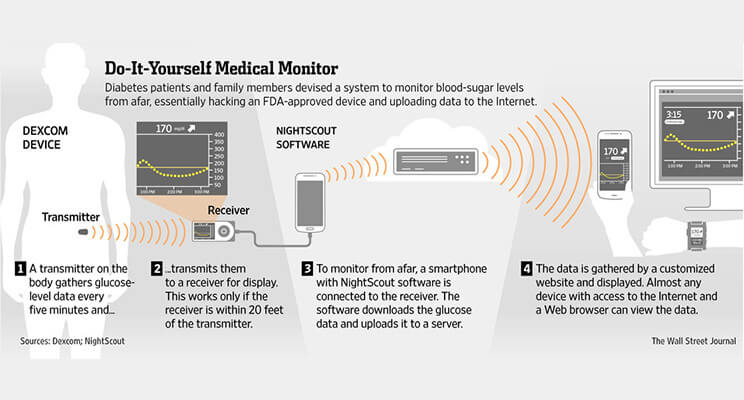
Much more concerning, however, is the blow CMS is striking against remote monitoring. Quite a few people with diabetes—including the elderly, the very young and those with hypoglycemia — really need a second pair of eyes on their blood sugar levels. So great was the need for this access that a community of parents and people with diabetes created their own remote monitoring solutions by hacking these devices to connect them to the cloud years before a commercial solution was available. Among them were two of my Bigfoot co-founders, Bryan Mazlish and Lane Desborough. Denying access to this kind of information sharing will put vulnerable lives at risk and escalate health care costs when people end up in the hospital unnecessarily.
Demand for remote glucose monitoring has been building since 2013, when the father of a young boy with diabetes refused to accept being left in the dark when his son went to school, unable to see what was happening with his son’s blood sugar levels. He developed software to access and transfer CGM data to the cloud so that it would be available to read whether his son was home or not.
This was the beginning of the Nightscout Project, which was soon joined by other people with diabetes and their loved ones, people who rallied under the slogan #WeAreNotWaiting.
As the movement grew, medical device companies paid attention. A few years later we have the first commercially available CGMs with sharing functions built in. This is a major victory.
That progress is now in jeopardy with the latest CMS decision.
I’m looking forward to the day when CMS reverses this decision, and I believe that day will come. Once CMS understands that no one is trying to argue that an app should be considered “durable medical equipment” the way a CGM receiver is. No one is trying to get the agency to buy smartphones for millions of Medicare beneficiaries.
Once we make this clear to them, and once they begin to understand that smartphone integration is a fundamental part of forthcoming innovations in digital health, they will begin to rethink this decision.
Patients of all ages and those who care for them are eager for innovations that will keep them safer and make their lives easier. Demand for automated insulin delivery with an easy-to-use smartphone patient interface will continue to increase as the digital health industry continues to grow and evolve.
It’s up to us, the technologists and innovators in health care, as well as providers and people with diabetes, to speak out against this recent CMS ruling. Whatever the agency’s rationale for cutting off the sharing lifeline, it’s up to us to remind them that lives and human health are at stake.